In 2014 biologist Mike Snyder published 42 papers. Some of his contributions to those papers were collaborative, but many describe experiments performed in his lab. To make sense of the number 42, one might naïvely assume that Snyder dedicated 8 days and 17 hours (on average) to each paper. That time would include the hours (days?) needed to conceive of ideas, setup and perform experiments, analyze data and write text. It’s not much time, yet even so it is probably an upper bound. This is because Snyder performed the work that resulted in the 42 papers in his spare time. As the chair of the genetics department at Stanford, an administrative position carrying many responsibilities, he was burdened with numerous inter- and intra- and extra- departmental meetings, and as an internationally renowned figure in genomics he was surely called upon to assist in numerous reviews of grants and papers, to deliver invited talks at conferences, and to serve on numerous national and international panels and committees. Moreover, as a “principal investigator” (PI), Snyder was certainly tasked with writing reports about the funding he receives, and to spend time applying for future funding. So how does someone write 42 papers in a year?
Snyder manages 36 postdocs, 13 research assistants, 11 research scientists, 9 visiting scientists, and 8 graduate students. One might imagine that each of the graduate students supervises four postdocs, so that the students/postdocs operate under a structure that enables independent work to proceed in the lab with only occasional supervision and input from the PI. This so-called “PI model” for biology arguably makes sense. The focus of experienced investigators on supervision allows them to provide crucial input gained from years of experience on projects that require not only creative insight, but also large amounts of tedious rote work. Modern biology is also fraught with interdisciplinary challenges, and large groups benefit from the diversity of their members. For these reasons biology papers nowadays tend to have large numbers of authors.
It is those authors, the unsung heroes of science, that are the “cast” in Eric Lander’s recent perspective on “The Heroes of CRISPR” where he writes that
“the narrative underscores that scientific breakthroughs are rarely eureka moments. They are typically ensemble acts, played out over a decade or more, in which the cast becomes part of something greater than what any one of them could do alone.”
All of the research papers referenced in the Lander perspective have multiple authors, on average about 7, and going up to 20. When discussing papers such as this, it is therefore customary to refer to “the PI and colleagues…” in lieu of crediting all individual authors. Indeed, this phrase appears throughout Lander’s perspective, where he writes “Moineau and colleagues..”, “Siksnys and colleagues..”, etc. It is understood that this means that the PIs guided projects and provided key insights, yet left most of the work to the first author(s) who were in turn aided by others.
There is a more cynical view of the PI model, namely that by running large labs PIs are able to benefit from a scientific roulette of their own making. PIs can claim credit for successes while blaming underlings for failures. Even one success can fund numerous failures, and so the wheel spins faster and faster…the PI always wins. Of course the mad rush to win leads to an overall deterioration of quality. For example, it appears that even the Lander perspective was written in haste (Lander may not be as prolific as Snyder but according to Thomson Reuters he did publish 21 “hot” papers in 2015, placing him fourth on the list of the world’s most influential scientific minds, only a few paces behind winner Stacey Gabriel). The result is what appears to be a fairly significant typo in Figure 2 of his perspective, which shows the geography of the CRISPR story. The circle labeled “9” should be colored in blue like circle “10”, because that color represents “the final step of biological engineering to enable genome editing”. The Jinek et al. 2012 paper of circle “9” clearly states that “We further show that the Cas9 endonuclease can be programmed with guide RNA engineered as a single transcript to target and cleave any dsDNA sequence of interest”. Moreover the Jinek et al. 2013 paper described editing in mammalian cells but was submitted in 2012 (the dates in Lander’s figure are by submission), so circle “9” should also be labeled with a “10” for “Genome editing in mammalian cells”. In other words, the figure should have looked like this:
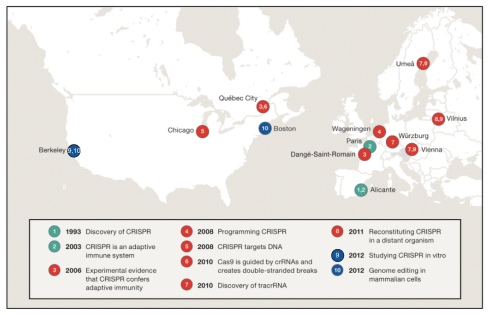
Lander acknowledges the reality of the PI model in the opening of his perspective, where he writes that “the Perspective describes an inspiring ensemble of a dozen or so scientists who—with their collaborators and other contributors whose stories are not elaborated here—discovered the CRISPR system, unraveled its molecular mechanisms, and repurposed it as a powerful tool for biological research and biomedicine. Together, they are the Heroes of CRISPR.”
But in choosing to highlight a “dozen or so” scientists, almost all of whom are established PIs at this point, Lander unfairly trivializes the contributions of dozens of graduate students and postdocs who may have made many of the discoveries he discusses, may have developed the key insights, and almost certainly did most of the work. For example, one of the papers mentioned in the Lander perspective is
F. Zhang*, L. Cong*, S. Lodato, S. Kosuri, G.M. Church and P. Arlotta, Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription, 2011
I’ve placed a * next to the names of F. Zhang and L. Cong as is customary to do when “authors contributed equally to this work” (a quote from their paper). Yet in his perspective Lander writes that “when TALEs were deciphered, Zhang, with his collaborators Paola Arlotta and George Church…successfully repurposed them for mammals”. The omission of Cong is surprising not only because his contribution to the TALE work was equal to that of Zhang, but because he is the same Cong that is first author of Cong et al. 2013 that Lander lauds for its number of citations (Le Cong is a former graduate student of Zhang, and is now a postdoc at the Broad Institute). Another person who is absent from Lander’s perspective, yet was first author on two of the key papers mentioned is Martin Jinek. He and Charpentier’s postdoc Krzysztof Chylinski were deemed fundamental to the CRISPR story elsewhere.
A proper narrative of the history of CRISPR would include stories primarily about the graduate students and postdocs who did the heavy lifting. Still, one might imagine giving Lander the benefit of the doubt; perhaps it was simply not possible to exhaustively describe all the individuals who contributed to the 20 year CRISPR story. Maybe Lander’s intent was to draw readers into his piece by including interesting backstories in the style of the New Yorker. Mentions of cognac, choucroute garnie and Saddam Hussein certainly added spice to what would otherwise be just a litany of dates. Yet if that is the case, why are the backstories of the only two women mentioned in Lander’s perspective presented as so so so boring? The entirety of what he has to say about them is “Charpentier had earned her Ph.D in microbiology from Pasteur Institute in 1995” and “Doudna had received her Ph.D. at Harvard” (those who are interested in Charpentier and Doudna’s fascinating CRISPR story will have to forgo the journal Cell and read about The CRISPR Quandary in the New York Times). A possible answer to my question is provided here.
Lander concludes his perspective by writing that “the [CRISPR] narrative…is a wonderful lesson for a young person contemplating a life in science”. That may be true of the CRISPR narrative, but the lesson of his narrative is that young persons should not count on leaders in their field to recognize their work.
COI statement: Eric Lander is my former (informal) Ph.D. advisor. Jennifer Doudna is a current colleague and collaborator.

19 comments
Comments feed for this article
January 18, 2016 at 1:26 pm
Rick Durrett
I usually enjoy your column but not when it degenerates into a personal attack
January 18, 2016 at 2:17 pm
James Webber
“Each graduate student supervises four postdocs” Ah, one can dream…
January 18, 2016 at 3:51 pm
Lior Pachter
For the record, the statement was intended tongue-in-cheek…as you say, something for students to dream about…
January 19, 2016 at 11:39 pm
Linus Schumacher
I very much enjoyed this line and the brief day-dream it inspired, thanks.
January 18, 2016 at 2:51 pm
Arnaud Martin
Landerification: a deliberate misrepresentation of ideas based on selective omission, name clumping, and claims of incrementation
e.g. Lander 2016, “The Heroes of AVIATION”
Icarus and Leonardo Da Vinci -> GREEN DOT Cailey/Ader/Lilienthal/Whitehead/Langley/The Wright Brothers -> RED DOT
Charles Lindberg -> BLUE DOT
January 24, 2016 at 5:53 pm
Claudiu Bandea
For and alternative, unconventional perspective on Lander’s article, please see my comment at PubMed Commons:
January 24, 2016 at 5:55 pm
Claudiu Bandea
http://www.ncbi.nlm.nih.gov/pubmed/26771483
January 24, 2016 at 8:24 pm
Arnaud Martin
Dear Claudiu, with all respects due, there is a reason why nobody has commented the Lander piece on PubMed Commons on the Lander Article (well, except Doudna and Charpentier), while there has been ample debate on other venues. I immediately had a dreadful feeling of ridicule reading your comment because it REALLY was obvious you tried to toot your own horn by providing irrelevant references. Good Luck
January 25, 2016 at 11:32 am
Claudiu Bandea
Thanks for your comment Arnaud. Just like you, I wondered why only Doudna and Charpentier have commented on Lander’s article in PubMed Commons. Apparently, you think “there is a reason”. Can you please share it with us? Thanks.
January 25, 2016 at 12:40 pm
Arnaud Martin
Hi Claudiu. Look at the brevity and humility of the Charpentier and Doudna comments: factual, non-verbose, directly relevant to the Lander article.
In contrast the reader of your comment has to go through a convoluted mental journey to connect thin dots to CRISPR.
And I still do not see how anything about jDNA that makes it comparable to an “adaptive immune system”, unlike CRISPR. The part about degenerative/prion diseases seemed equally irrelevant. A key difference between the two phenomena you mentioned and CRISPR is the lack of (knowledge about) specific targeting.
While I value provocative ideas in science, your Pubmed Commons comment felt misplaced and it is all I wanted to say – I have no expertise to really evaluate the validity of your claims in the BioRxiv pre-prints
January 25, 2016 at 2:28 pm
Claudiu Bandea
Hi Arnaud,
You stated in your previous comment: “…there is a *reason* why nobody has commented the Lander piece on PubMed Commons on the Lander Article (well, except Doudna and Charpentier), while there has been ample debate on other venues”.
You are obviously right, so what’s the reason you had in mind?
January 25, 2016 at 11:51 am
Claudiu Bandea
Arnaud,
About the rest of your comment, YES it is OBVIOUS that I’m bringing forward two scientific issues that I have been working on for a long time, but at least they are about science and the health of millions of people (i.e. not about ‘money’, ‘egos’, and ‘ prizes’), which I think should be a welcomed intervention :).
Regarding your point about my references being irrelevant, I think you missed the association I have tried to make between the CRISPR story and the issues I brought forward. So, please let me elaborate on this association and its potential relevance.
As you knows, CRISPR is an adaptive immune system that protects the hosts from viruses, and it does that by using previously co-opted viral sequences. As you also know, the restriction/modification system as well as the microRNA also evolved as defensive immune systems. All of these initially mysterious phenomena, as well and many others that I have not mentioned in my PubMed Commons comment, including the evolution of antibodies, T-cells receptors and the MHC system, are the result of the never-ending co-evolution between viruses and their hosts. Let me mention one more fascinating discovery in biology, the fact that the ‘introns’ and spliceosomes have evolved as a genomic immune system against insertional mutagenesis by viral elements.
So, if there is a lesson to be learned from all of these great discoveries, is that the selection pressure conferred by viruses has led to the evolution of numerous defense immune systems, some of which have yet to be recognized. So, to me, it would make sense to search for additional putative defense systems among the mysterious biological phenomena that have remained enigmatic despite decades of research and thousands of publications.
One such phenomenon is the C-value enigma, which has been studied for more than half-of-a-century, but remains one of the greatest unanswered questions in biology. Obviously, this lack of knowledge is not something to be proud of (to say the least), so there is an urgent need for new paradigms that are consistent with the vast amounts of data in the field, and make biological and evolutionary sense. We know that most of our genome is composed of viral sequences and their evolutionary derivatives, which currently are referred to as “junk DNA”. As I mentioned above, we also know that the ‘introns’, which represent a large portion of the so called “junk DNA”, have evolved as a protective mechanism against insertional mutagenesis by viral elements. So, why it is irrelevant to hypothesize that, just like CRISPR, the so called “junk DNA” serves as an adaptive genomic immune system against insertional mutagenesis, which might explain the C-value enigma and the evolution of genome size?
I think that the other putative innate immune system that I brought forward is even more important because it concerns a group of mysterious diseases, including Alzheimer’s, Parkinson’s, Huntington’s, ALS, and Creutzfeldt-Jakob disease, that affect the life of tens of millions of people worldwide, and cost us hundreds of billions of dollars every year. What you and many other readers might not know is that despite decades of research, thousands of studies, and numerous advances, the etiology of Alzheimer’s, Parkinson’s, Huntington’s, ALS, and Creutzfeldt-Jakob disease is not known, and there are no successful preventive or therapeutic approaches. Moreover, the physiological function of APP/amyloid-beta, tau, alpha-synuclein, huntingtin, TAR DNA-binding protein 43 and prion protein, which are among the most studied proteins in the world, is not known. Isn’t this fact hard to accept? Isn’t this blatant lack knowledge a clue that something is not right about the direction of the research in the field? I think the model I proposed on the biological function of these proteins as members of innate immune system, and on the etiology of this group of diseases makes biological and evolutionary sense and, therefore, it should be openly and timely evaluated. The OBVIOUS question is, of course, why the investigators in the field (which, BTW, are not to be blamed for the lack of progress) are hesitant to openly and timely evaluate this model, and either dismiss it or embrace it?
January 25, 2016 at 12:16 pm
Claudiu Bandea
In my comments above, I referred to my PubMed Commons mini-essay and the associated references, so to facilitate reading I’m copying them here:
Unconventional Lessons from “The Heroes of CRISPR”
With an Altmetric score of well over 1000 at the time of posting this comment, “The Heroes of CRISPR” backstory by Eric Lander (1) has been devoured by thousands of scientists, laymen, …and lawyers (2). Much of the response, encompassing factual corrections, disclaimers, and piercing tweets, has followed ‘the money and prizes’ (2). Here is an alternative perspective.
I think the true heroes of CRISPR are the microbial organisms, which have invented this fascinating immune system over millions of years of evolution and could not have done it without the contribution of viruses. Should these evolutionary heroes be co-applicants on the CRISPR patents?
Not too long ago, microorganisms were the unsung heroes of another highly regarded and prized human achievement, the restriction modification immune system, which led to the recombinant DNA era. And, just a few years ago, the microRNA platform, an antiviral defense system eventually co-opted as a gene-expression regulatory mechanism, was as exciting, promising, and prized as CRISPR. So, what other enigmatic phenomena born from the eternal co-evolution of viruses and their hosts are there in the waiting to be recognized as defense immune systems?
If the CRISPR publishing fiasco reported by Lander (1) is correct, then we should search among stories that are having a hard time making into published science or penetrating the formidable protective wall surrounding conventional science. Let me point out a couple of them:
(i) What if most of our genome, which we know is composed primarily of viral sequences and their remnants, is not “junk DNA” as traditionally perceived, but serves, just like CRISPR, as an adaptive immune system against insertional damage, particularly against insertional mutagenesis leading to cancer, by endogenous and exogenous viral elements (3)?
(ii) What if APP/amyloid-beta and tau, alpha-synuclein, huntingtin, TAR DNA-binding protein 43, and prion protein, the primary proteins implicated in Alzheimer’s, Parkinson’s, Huntington’s, ALS, and Creutzfeldt-Jakob Disease, respectively, whose biological function has remained mysterious despite decades of research and thousands of studies, are members of the innate immune system (4)? In the United States alone, these diseases affect more than 5 million people and their families, cost in the hundreds of billions of dollars per year, and cause unmeasurable suffering. Is this medical, economic, and social burden high enough to prompt an open evaluation of this theory, which is well supported (see Ref. 5 for the latest evidence) but challenges the scientific legitimacy of the highly celebrated and prized working hypotheses in the field, the ‘protein misfolding’ and ‘prion’ paradigms?
References:
(1) Lander ES. 2016. The Heroes of CRISPR. Cell. 164(1-2):18-28
(2) Altmetric citations: http://www.sciencedirect.com/science/article/pii/S0092867415017055
(3) Bandea CI. 2013. On the concept of biological function, junk DNA and the gospels of ENCODE and Graur *et al.* BioRxiv: http://biorxiv.org/content/biorxiv/early/2013/11/18/000588.full.pdf
(4) Bandea CI. 2013. Aβ, tau, α-synuclein, huntingtin, TDP-43, PrP and AA are members of the innate immune system: a unifying hypothesis on the etiology of AD, PD, HD, ALS, CJD and RSA as innate immunity disorders. BioRxiv: http://biorxiv.org/content/biorxiv/early/2013/11/18/000604.full.pdf
(5) Beatman EL et al. 2015. Alpha-synuclein expression restricts RNA viral infections in the brain. J. Virol. (Epub ahead of print);
January 25, 2016 at 9:25 pm
Mike Lassner
I would submit that the step 10 circle should encompass the entire globe–it was done by dozens labs in cities all over the world–not just Boston and Berkeley. After Vilnius and Berkeley groups showed that CAS9 target site specificity could be programmed via guide RNA sequence, dozens of groups all over the world rapidly demonstrated that it could be used in all kinds of eukaryotic cells. The Boston labs (as all but FZ’s lab admit), were major players in making improvements to the practice, but the major reason they published a few months ahead were: (1) they had ready to go toolboxes using TALENS and ZFNs ready to plug in a new reagent and (2) they had the prestige to get published more rapidly than others.
January 27, 2016 at 10:50 am
Claudiu Bandea
Arnaud,
I’m still hopping you’ll reveal the ‘reason’ you think very few people have commented on Lander’s article at PubMed Commons, but until then, here’s my response to the rest of your comment.
Regarding your remark “Look at the brevity and humility of the Charpentier and Doudna comments: factual, non-verbose, directly relevant to the Lander article”, definitely, their comments were very brief, perhaps too brief to be complete; see Lander’s subsequent disclosures at Cell’s website on he’s communications before the publication of his article. Also, Doudna’s and Charpenter’s comments addressed communication technicalities not scientific issues, which I think is the main purpose of PubMed Commons.
I don’t know about you, but I really appreciated Lior’s ‘verbose’ take on Lander’s article, which he started with two full paragraphs on Mike Snyder’s 42 papers production in 2014 and on his outrageously large entourage of *working class scientists*. Too bad that Lior didn’t introduce us directly to one of CRIPSR’s heroes by mentioning Doudna, who according to New-York Time is “the head of a formidably large lab at the University of California, Berkeley” (http://www.nytimes.com/2015/11/15/magazine/the-crispr-quandary.html). Is this description of Doudna’s lab correct, Lior? In any case, I thought Lior’s incursion in the ‘PI model’ for conducting science, albeit ‘verbose’, was relevant to the CRISPR story and, more generally, to the increasing dichotomy between the *elitists* and the *working class* in science.
About the “humility” you attributed to Charpentier and Doudna, didn’t one of them recently said something about inventing CRISPR?
Regarding my comment in PubMed Commons, indeed it was rather convoluted, particularly for people who had no interest in reading the references I posted. I’ll probably rectify that in another PubMed Commons, but until then you might want to read a comment I just posted at Michael Eisen’s blog, “it is NOT junk”, in which I hope I presented the case more clearly (http://www.michaeleisen.org/blog/?p=1825#comment-1502780).
Nevertheless, to grasp the relevance of my association, I think you need to read my BioRxiv references. I have no doubt that you have the “expertise” to evaluate them; if you don’t have it, then, who has it? Please, let me explain. When I ask researchers in the field of neurodegenerative diseases to evaluate the theory, they say they don’t have the expertise in viral co-evolution with their hosts, or in immunology. When I ask immunologists, they apparently don’t have the expertise in neurodegenerative diseases, viruses, or evolution. When I ask virologists and evolutionists, it seems, they don’t have expertise in neurodegenerative diseases and immunology, etc.
Lior,
Sorry to put you on the spot, but please let me ask you is, as a computational biologist, you have the expertise to address the hypothesis that so call “junk DNA” serve as an (adaptive) genomic defense system against insertional mutagenesis by endogenous and exogenous viral events? Particularly, it would be helpful to know what you think about the key tenet of this theory, that in humans, for example, who have over 30 trillion of rapidly dividing cells, the so called “junk DNA” protects against insertional mutagenesis leading to cancer. I know it’s a rather complicated mathematical undertaking, but if you can’t evaluate it, who can?
January 28, 2016 at 5:31 am
Anonymous
Arnaud,
I’m still hopping you’ll reveal the ‘reason’ you think very few people have commented on Lander’s article at PubMed Commons. Until but until then, here’s my response to the rest of your comment.
Regarding your remark “Look at the brevity and humility of the Charpentier and Doudna comments: factual, non-verbose, directly relevant to the Lander article”, definitely, their comments were very brief, perhaps too brief to be complete; see Lander’s subsequent disclosures at Cell’s website on he’s communications before the publication of his article. Also, Doudna’s and Charpenter’s comments addressed communication technicalities not scientific issues, which I think is the main purpose of PubMed Commons.
I don’t know about you, but I really appreciated Lior’s ‘verbose’ take on Lander’s article, which begins with two full paragraphs on Mike Snyder’s 42 papers production in 2014 and on his outrageously large entourage of *working class scientists*. Too bad that Lior didn’t bring forward one of CRIPSR’s heroes by mentioning Doudna, who according to New-York Time is “the head of a formidably large lab at the University of California, Berkeley” (http://www.nytimes.com/2015/11/15/magazine/the-crispr-quandary.html). In any case, I thought Lior’s incursion in the ‘PI model’, albeit ‘verbose’, was highly relevant to the CRISPR story and, more generally, to the increasing dichotomy between the *elitists* and the *working class* in science.
About the humility you attributed to Charpentier and Doudna, didn’t one of them said something about inventing CRISPR?
Regarding my comment in PubMed Commons, indeed it was rather convoluted, particularly for people who had no interest in reading the references I posted. I’ll probably rectify that in another PubMed Commons, but until then you might want to read a comment I just posted at Michael Eisen’s blog, “it is NOT junk”, in which I hope I presented the case more clearly (http://www.michaeleisen.org/blog/?p=1825#comment-1502780).
Nevertheless, to grasp the relevance of my association, you need to read the 2 BioRxiv references. I have no doubt that you have the “expertise” to evaluate them; if you don’t have it, then, who has it? Please, let me explain. When I ask researchers in the field of neurodegenerative diseases to evaluate my theory on the etiology of these diseases, they say they don’t have the expertise in viral co-evolution with their hosts, or in immunology. When I ask immunologists, apparently don’t have the expertise in neurodegenerative diseases, viruses, or evolution. When I ask virologists and evolutionists, it seems, they don’t have expertise in neurodegenerative diseases and immunology, etc.
Lior,
Sorry to put you on the spot, but please let me ask you if, as a computational biologist, you have the expertise to address the hypothesis that so call “junk DNA” serve as an (adaptive) genomic defense system against insertional mutagenesis by endogenous and exogenous viral events? Particularly, it would be helpful to know your thoughts on the key tenet of this theory, that in humans, for example, who have over 30 trillion of rapidly dividing cells, the so called “junk DNA” protects against insertional mutagenesis leading to cancer. This a rather complicated mathematical undertaking, but if you can’t evaluate it, who can? Thanks.
January 30, 2016 at 7:08 am
12jrowley2
In actuality, since PI’s are often given credit for just funding work and providing lab space, the funding institution should get the credit. Going farther maybe we should say that the buddy’s of the PI on the study section who gave the funding to (rather than to the real underling investigators who they stole the data from to write the grant) should get the credit.
January 31, 2016 at 10:58 am
Donald Forsdyke
HYPOTHESIS-DRIVEN RESEARCH
The discoveries of a cytosolic microbial adaptive immune system (CRISPR) and its applications to genome editing are major scientific advances. A review of the history of this magnificent achievement, made mainly by young people close to those with abundant research funds, is welcome. But the implication that this history supports the non-hypothesis-driven approach to research is questionable.
Backed by inexpensive bioinformatic analyses, a hypothesis of cytosolic innate immunity was developed in the 1990s [1-3]. Had this CRISPR-analogous hypothesis been backed by funding, CRISPR and its applications might have been achieved more expeditiously. Thus, there are many roads to Rome. Because the well-equipped army that took route A arrive first, it does not follow that route A is superior to route B. Likewise, this comment could have been written in prose or poetry. Your liking (perhaps) of the present prose rendition, does not disprove the proposition that a poetic version might have been superior.
[1] Forsdyke & Mortimer (2000) Chargaff’s legacy. Gene 261, 127-137.Forsdyke DR, 2000
[2] Cristillo et al. (2001) Double-stranded RNA as a not-self alarm signal: to evade, most viruses purine-load their RNAs, but some (HTLV-1, Epstein-Barr) pyrimidine-load. J Theor Biol 208, 475-491.Cristillo AD, 2001
[3] Forsdyke, Madill & Smith (2002) Immunity as a function of the unicellular state: implications of emerging genomic data. Trends Immunol 23, 575-579.Forsdyke DR, 2002
[This is another PubMed Commons comment. I also submitted a poem which the PubMed Commons authorities are currently pondering as to admissibility into their domain.]
May 15, 2016 at 3:54 am
Amir Feizi (@feizi_a)
Thanks Pachter! I see all these writings is just decorating a simple man aged rule: “being a skilled person (principal investigator) is very tough, but it is extremely tougher to stay and be fair when you enter to the game of fame and money knowing you can beat everyone if you want!